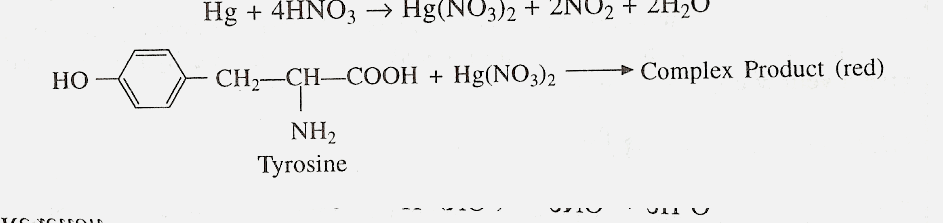Amino Acids and Proteins
Introduction
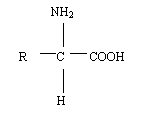
Amino acids are organic
compounds that contain amino and carboxyl group and therefore possess both
basic and acidic properties. Proteins are polymer of amino acids and contain
about 22 different amino acids, with the exception of proline and
hydroxyproline, which strictly speaking are amino acids. General formula of
an amino acid is as shown below:
All amino acids with the
exception of glycine show optical activity due to presence of asymmetric
carbon atom
Protein (Greek:
Proteios, meaning holding first place) are one of the most important group
of biomolecules. The quantity and type of protein shows a wide variation
between not only among different organsims but also is different organs or
parts of the same organism. Proteins perform very vital and diverse roles in
organisms. A simple bacterial cell such as Escherichia coli contains more
than 5000 different type of proteins.
QUALITATIVE TESTS FOR AMINO
ACIDS AND PROTEINS
The various qualitative tests
for detection of amino acids in a sample preparation are described.
A) NINHYDRIN TEST
Principle
The test due to a reaction
between a -amino group of free amino acid and ninhdyrin. Ninhydrin is a
powerful oxidising agent and in its presence, amino acids undergo
oxidative deamination liberating ammonia, carbon dioxide, a corresponding
aldehyde and reduced from of ninhydrin. The ammonia formed from a -amino
group reacts with ninhydrin and its reduced product to give a blue
substances diketohydrin. However, in case of imino acids like proline, a
different product having a bright yellow colour is formed. Asparagine
which has a free amide group reacts to give a brown coloured product. This
test is also given by proteins and peptides.
Reaction

Materials and Reagents
1. Boiling water bath
2. Ninhydrin: 0.2% solution
prepared in acetone.
3. Test solution: Prepare
solution containing 50 m g/ml of individual amino acids
Procedure and Observation
Add 2-5 drops of ninhydrin
solution to 1 ml of test solution or sample preparation. Mix and keep for
5 min in boiling water bath and observe the development of a pink, purple
or violet-blue color. Imino acids proline and hydroxyproline give a yellow
colored complex.
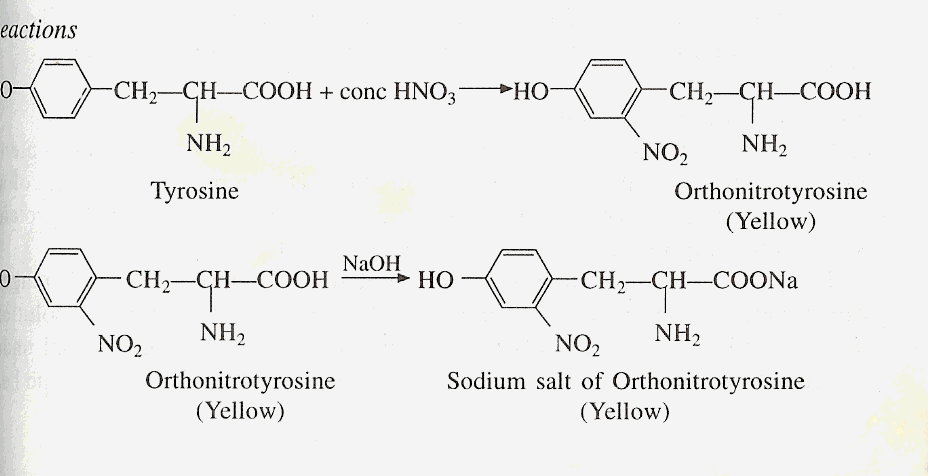
B) XANTHOPROTEIC TEST
Principle
Amino acids containing an
aromatic nucleus from yellow nitro derivatives on heating with HNO3.
The salts of these derivatives are orange in color. Proteins containing
these amino acids give a positive response to this test.
Reaction
Materials and Reagents
1. Conc HNO3.
2. NaOH solution : Dissolve
40 g of NaOH in water and make the final volume to 100 ml.
3. Test solutions: Prepare
solutions containing 50 m g/ml of amino acids like tyrosine, glycine,
tryptophan, phenylalanine, lysine, cysteine, eucine etc.
Procedure and observations
Add 1ml conc HNO3
into ml of test solution. Cool it and then slowly pipette NaOH till the
solutions alkaline. Note weather the mixture turns orange red in colour.
Apperance of orange red colour denotes presence of aromatic amino acids.
C) MILLONíS TEST
Principle
Amino acids or compounds
containing hydroxybenzene radical react with Millonís regents which
constitutes of mercurous and mercuric nitrates containing HNO3,
to form a red complex. Thus this test is specific for tyrosine.
Reaction
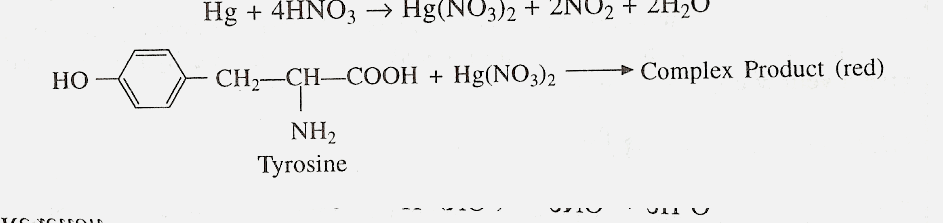
MATERIALS AND REAGENTS
i) Boiling water
bath
ii) Millonís reagent:
Dissolve 10 g of Hg metal in 20 ml of conc HNO3, dilute to 100
ml with water after cessation of evolution of the brown fumes.
iii) Test solutions of
different amino acids made as in the preceding test. Use a solution of
Phenol also as a test sample.
Procedure and Observations
Add 2-5 drops of Millonís
reagent into 1 ml of test solution. Shake the contents and place the test
tubes in boiling water bath. Development of a red color indicates presence
of tyrosine. A positive reaction will also be obtained for proteins, which
contain tyrosine.
The same experiment may be
carried out a modified Millonís reagent which contains 10% mercuric
sulphate in 10% H2SO4. To 1 ml of test solutions,
add 1 ml of modified Millonís reagent and boil gently for 1 min. Cool
under running tap water and then add one or two drops of 1 % NaNO2
solutions and heat slightly. Appearance of red color denotes presence of
tyrosine. The advantage of using modified Millonís test lies in its being
less susceptible to interference from inorganic salts.
D) HOPKINS-COLE TEST
Principle
The indole group of
tryptophan reacts with glyoxylic acid in the presence of conc. H2SO4
to give a purple color. Glyoxylic acid is prepared by reducing oxalic acid
with magnesium powder or sodium amalgam. Glacial acetic, which has been
exposed to the sunlight also, contains glyoxylic acid and can thus be used
for this test.
Reaction
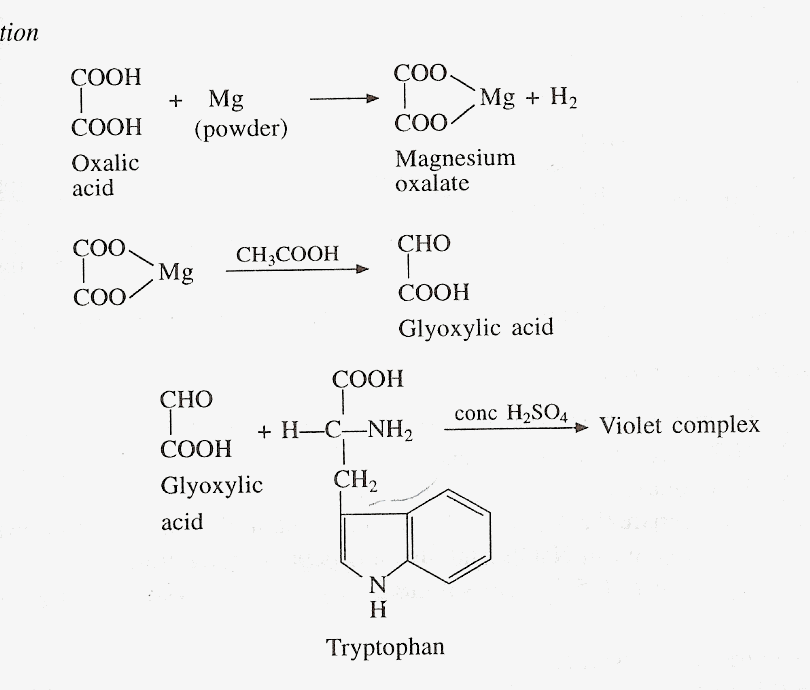
Materials and Reagents
1. Test reagent: To
10 g of magnesium powder add enough water to submerge it. Then slowly add
250 ml of cold saturated oxalic acid solution. Keep cool. Filter magnesium
oxalate formed. Acidify the filtrate with 25 ml of glacial acetic acid and
dilute it to 1 L with water. Alternatively, glacial acetic acid, which has
been kept in sunlight for a few minutes may be used as a test solution.
2. Test solutions:
prepare solutions containing 50-100m g/ml of individual amino acids such
as tryptophan, tryosine, phenylalnine, glutamic acid etc.
3. Conc H2SO4
Procedure and Observations
Add 1 ml of Hopkins-Cole
test reagents to 1 ml of test solution. Mix and pour with pipette conc. H2SO4
along the sides of test tube. Observe for appearance blue or violet ring
at the interface of the liquids, which would indicate presence of
tryptophan.
E) BIURET TEST
Principle
This test is a general test
compounds having a peptide bond. Alkaline copper sulphate reacts with
compounds containing two or more peptide bonds to give a violet or pinkish
coloured product which is due to formation of coordination complex of
cupric ions with unshared electron pairs of peptide nitrogen and oxygen of
water.
Reaction
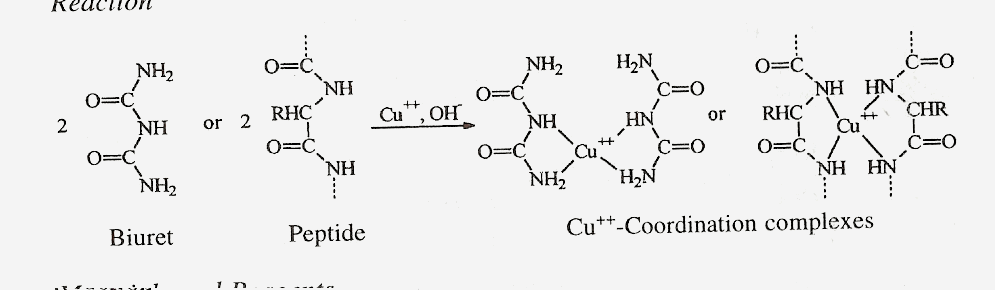
Materials and Reagents
1. Copper sulphate: 1% of
CuSo4.5H2O in water.
2. 40% solution of NaOH.
3. Test solutions:
a) Prepare 0.5% solution of
a protein like casein or bovine serum albumin in NaOH and also a
tripeptide glutathione.
b) Prepare 0.5% solutions
of glutamate, cysteine and glycine.
Procedure and observations
Add 0.5 ml NaOH to 1 ml of
test solutions and mix well. Then add 2-5 drops of copper sulphate and
observe for the formation of pink or violet colour. Appearance of pink or
violet colour shows presence of petides or proteins in the sample. Try
this test with solutions of glutathione and its constituent amino acids
and record the observations.
F) PAULYíS TEST
Principle
Diazotized sulphanilic acid
couples with amines, phenols and imidazole to form highly coloured
compounds. The diazonium compound is only formed in the cold, so all
solutions must be cooled in ice before diazotization.
Reaction
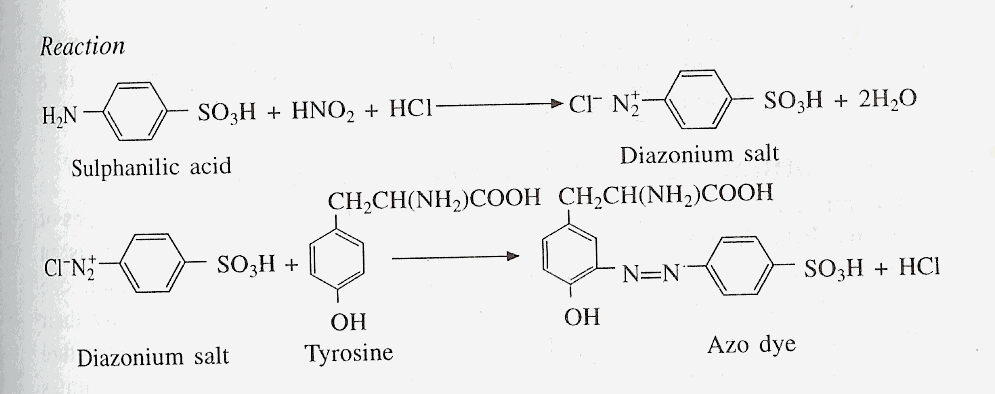
Materials and Reagents
1. Amino acids: 0.1%
solutions of glycine, tyrosine, histidine, tryptophan.
2. Sulphanilic acid: Make
1% solution of sulphanilic acid in 10% HCl.
3. 5% sodium nitrite
solution in water.
4. 1% solution of sodium
carbonate in water.
Procedure and observations
Mix 1 ml of sulphanilic
acid with 2 ml of the test solutions cooled in ice, add 1 ml of sodium
nitrite and leave in the cold for 3 min. make the solution alkaline by the
addition of 2 ml of sodium carbonate solution. Note whether any coloured
products are formed or not.
G) SAKAGUCHI REACTION
Principle
This reaction is given by
guanidinium compounds. The only amino acid containing the guanidine group
is arginine, and this reacts with a -naphthol and an oxidizing agent such
as bromine water or sodium hypochlorite to give a red coloured product.
The other guanidine containing non-amino acid compounds also give this
reaction.
Materials and Reagents
1. Amino acids: 0.1%
solution of amino acids like glycine, arginine, lysine, tyrosine etc.
2. Guanidines: 0.1% solution of
glycocyamine, methyguanidine and creatine.
3. 0.1% urea solution.
4. NaoH: 40% (w/v)
5. a -Naphthol: 1% in alcohol.
6. Bromine water: Add a few drops of
bromine to 100 ml water and shake. This should be done in a fume chamber.
Procedure and observations
Mix 1 ml of NaOH with 3 ml
of the amino acid or test solution and add 2 drops of a -naphthol. Mix
thoroughly and add 4 to 5 drops of bromine water. Watch for the formation
of red color which indicate presence of arginine or a guanidinium
compound.