
Qualitative Tests for Carbohydrates
Potato consists of different carbohydrates like starch, reducing sugars etc. Difficulties are encountered in the qualitative and quantitative analysis of samples containing mixtures of carbohydrates, particularly the sugars, because of their structural and chemical similarity and also with respect to their stereoisomers. During biochemical investigations it may because necessary to establish whether a given sample, particularly of a purified preparation, consist carbohydrates or not. Several rapid tests are available the presence or absence of a sugar or a carbohydrate in a sample. These tests are based on specific colour reactions typical for their group and are described below. For laboratory practical, it may be advised to perform these tests with the individual rather than mixture of sugars. Use of sugar solutions of different concentrations (0.1-1%) during these experiments would also provide valuable information about the sensitivity of these tests. The types of carbohydrates detected by these tests are:
Name of the test Application
Molisch’s Test General test for carbohydrates
Anthone Test General test for carbohydrates
Iodine Test For glycans (starch, glycogen)
Barfoed’s Test To distinguish between mono-saccharides from reducing diasaccharides
Seliwanoff’s Test For Ketones
Fehling’s Test For reducing sugars
Bendict’s Test For reducing sugars
Picric acid Test For reducing sugars
Bial’s Test For pentoses
1) MOLISCH’S TEST
Principle
This is a general test for all carbohydrates. Conc. H2SO4 hydrates glycosidic bonds to yield monosaccharides which in the presence of an acid get dehydrated to form furfural and its derivatives. These products react with sulphonated α-naphthol to give a purple complex. Polysaccharides and glycoproteins also give a positive reaction.
Reaction

Reagents
1. Conc. H2SO4
2. α-naphthol: 5% (w/v) in ethanol (prepare fresh)
Procedure and observations
Add 2-3 drops of α-naphthol solution to 2 ml of the test solution. Very gently pipette 1ml conc. H2SO4 along the side if the test tube so that the two distinct layers are formed. Carefully observe any color change at the junction two layers. Appearance of purpose color indicates the presence of carbohydrates in the sample preparation or the test solution.
Precautions
-naphthol solution is unstable and should be prepared fresh.1. α
2. Conc. H2SO4 should be along the sides of the test tubes causing minimal disturbance to the contents in the tube.
2) ANTHRONE TEST
Principle
Anthrone reaction is another general test for carbohydrates. In this the furfural produced reacts with anthrone to give bluish green colored complex.
Reaction
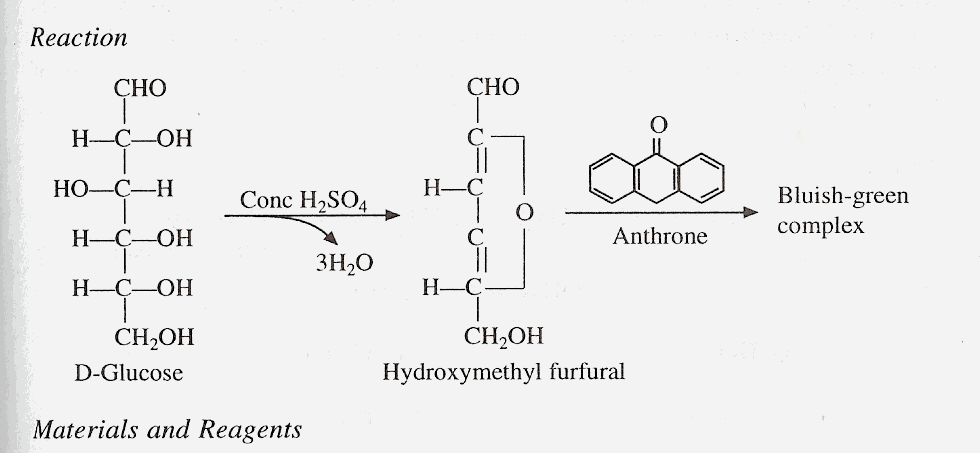
Materials and Reagents
Procedure and observations
Add 0.5 - 1 ml of the test solution to about 2 ml of anthrone reagent and mix thoroughly. Observe whether the color changes to bluish green. If not, examine the tubes again keeping them in boiling water bath for 10 min.
3) IODINE TEST
Principle
Iodine forms colored adsorption complexes with polysacchaides. Starch gives blue color with iodine, while glycogen reacts to form reddish brown complex. Hence it is useful, convenient and rapid test for detection of amylase, amylopectin and glycogen.
Reagents
Procedure and observations
Take 1 ml of the sample extract or test solution in a test tube. Add 4 - 5 drops of iodine solution to it and mix the contents gently. Observe if any colored product is formed. Note the color of the product.
4) Barfoed’s Test
Principle
This test is used for distinguishing monosaccharides from reducing disaccharides. Monosaccharides usually react in about 1 - 2 min while the reducing disaccharides take much longer time between 7 - 12 min to get hydrolysed and then react with the reagent. Brick red color is obtained in this test which is due to the formation of cuprous oxide.
Reaction
(CH3COO)2Cu2 + H2O ® 2CH3COOH + Cu(OH)2
Cupric acetate Cupric hydroxide
Cu(OH)2 ® CuO+H2O
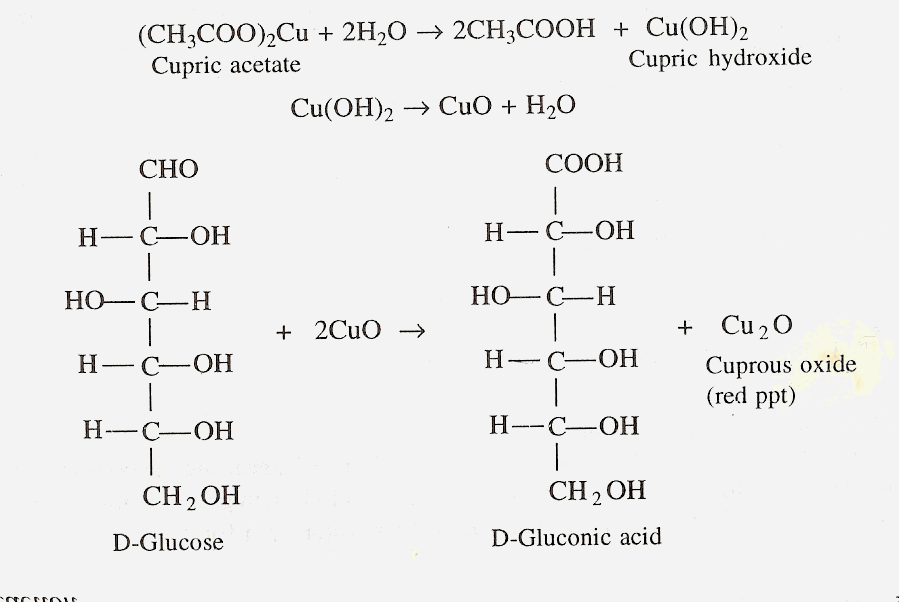
Materials and Reagents
Procedure and observations
Take 2 ml of Barfoed’s solution in a test tube and add 1ml of sample solution to it. Keep the test tubes in a boiling water bath. A briskly boiling water bath should be used for obtaining reliable results. Look for the formation of brick red color and also note the time taken for its appearance.
5) SELIWANOFF’S TEST
Principle
This test is used to distinguish aldoses from ketoses. Ketoses undergo dehydration to give furfural derivatives, which then condense with resorcinol to form a red complex. Prolonged heating will hydrolyze disaccharides and other monosaccharides will also eventually give color.
Reaction
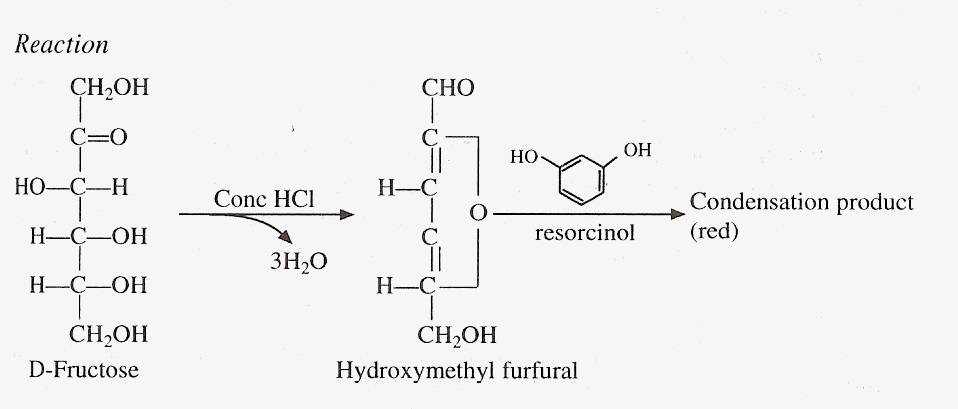
Materials and Reagents
- Boiling water bath
- Seliwanoff’s reagent: 0.05% (w/v) resorcinol in 3 HCl
Procedure and observations
Add 1ml of the test solution to 2 ml of Seliwanoff’s reagent and warm in a boiling water bath for 1min. Note for the appearance of a deep red color. This would indicate that the sample solution contains a keto sugar.
6) Fehling’s Test
Principle
Fehling’s test is a specific and highly sensitive for detection of reducing sugars. Formation of yellow or red ppt of cuprous oxide denotes the presence of reducing sugars. Rochelle salt acts as the chelating agent in this reaction.
Reaction
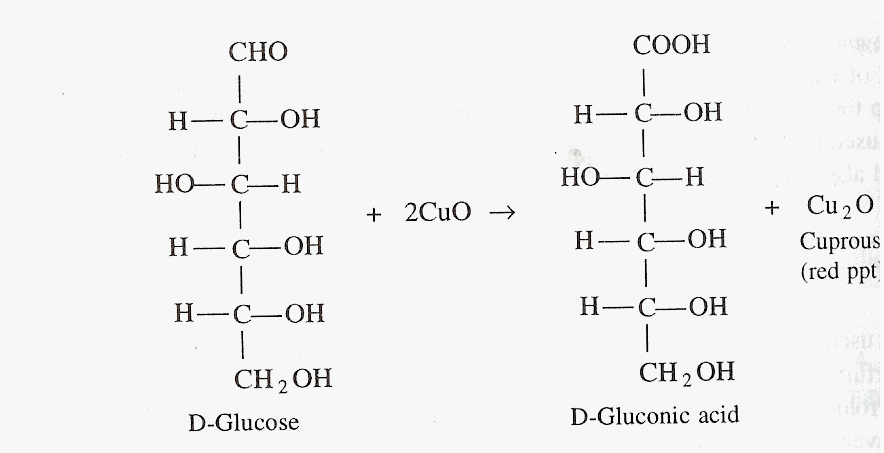
Materials and Reagents
- Boiling water bath.
- Fehling’s solution A: Dissolve 35 g of CuSO4.5H2O in water and make the volume to 500 ml.
- Fehling’s solution B: Dissolve 120 g of KOH and 173 g Na-K tartrate (Rochelle salt) in water and make the volume to 500 ml.
- Fehling’s reagent: Mix equal volumes of Fehling’s solution A and B. These solutions must be mixed immediately prior to use.
Procedure and observations
Add 1 ml of Fehling’s reagent (Reagent No. 4) to 1 ml of aliquot of the test solution. Mix thoroughly and place the test tubes in vigorously boiling water bath. Look out for the formation of red ppt of cuprous oxide which would indicate the presence of reducing sugars in the solution.
7) Benedict’s test
Principle
Benedict’s test is more convenient and this reagent in more stable. In this method sodium citrate functions as a chelating agent. Presence of reducing sugars results in the formation of red ppt of cuprous oxide.
Reaction
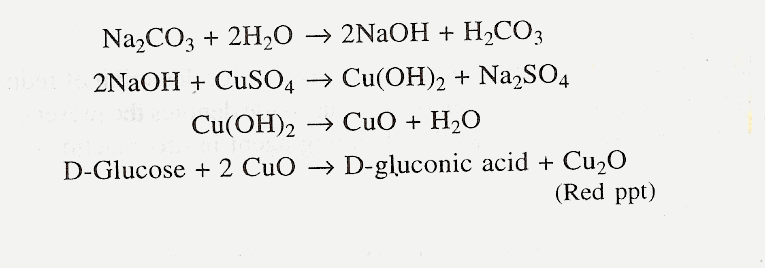
Materials and Reagents
Procedure and observations
Add 0.5 - 1 ml of the test solution or sample extract to 2 ml of Benedict’s reagent (Reagent No. 4). Keep the test tubes in a vigorously boiling water bath. Observe for the formation of red precipitates whose appearance would suggest the presence of reducing sugars in the given or sample extract.
8) Picric acid test
Principle
It is another test for detection of reducing sugars. The reducing sugars react with picric acid to form a red colored picramic acid.
Reaction
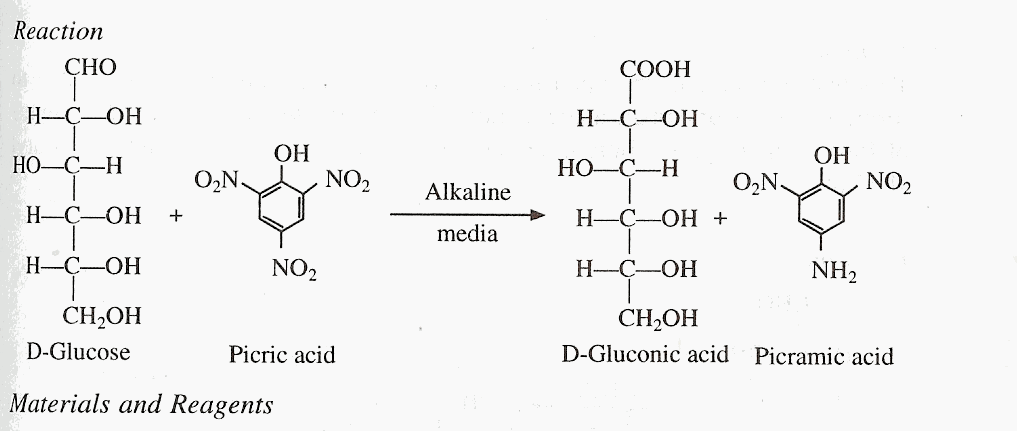
Materials and Reagents
Procedure and observations
Add 1 ml saturated picric acid to 1 ml of sample solution followed by 0.5 ml 10% Na2CO3. Heat the test tubes in a boiling water bath. Appearance of red color would indicate the presence of reducing sugars in the sample solution.
9) Bial’s test
Principle
This test is useful in the determination of pentose sugars. Reaction is due to formation of furfural in the acid medium which condenses with orcinol in presence of ferric ions to give a blue-green colored complex which is soluble in butyl alcohol.
Reaction
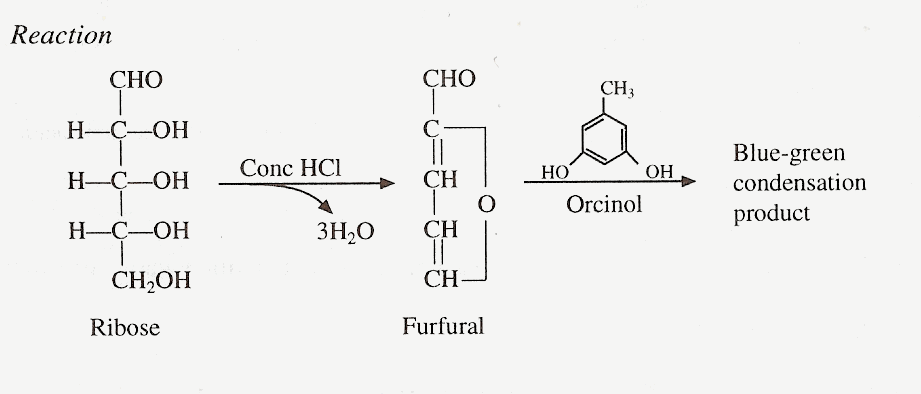
Materials and Reagents
Procedure and observations
To 2 ml of Bial’s reagent add 4-5 drops of test solution and heat in a boiling water bath. Observe for the formation of blue-green colored complex.