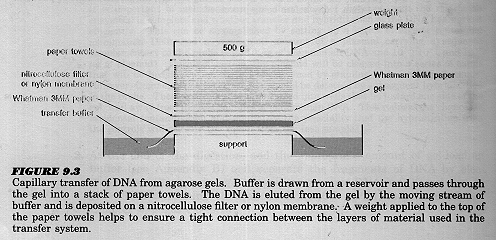
Southern Blot Hybridisation to determine the copy number and
integration of the transgene
Day 1:
Pre-hybridization and hybridization of blot
(this protocol assumes ~300 cm2 size blot)
- Preheat ~50 ml of hybridization buffer to
~60 C in the hybridization oven.
- Pour hybridization buffer into a plastic
container (slightly larger than the blot). Submerge blot in the hybridization
buffer, blot should be completely wet.
- Roll up blot (DNA side "inside") and place
in a hybridization tube. Pour hybridization solution from the container to the
hybridization tube. Secure cap. Unroll blot inside the tube by rotating the
tube.
- Place tube with blot into the hybridization
oven and incubate at 60 C for 30 min.
- Prepare salmon sperm DNA: place 250 micro
litre in a screw cap microfuge tube, incubate in a boiling water bath or heat
block for 5 min. then on ice for 5 min.
- After the 30 min. 60 C incubation period
for the blot (see step 21), add the 250 micro litre denatured salmon sperm DNA
to the hybridization buffer in the tube. Incubate the blot for an additional
hour at 60 C.
Prepare probe for labeling:
- Prepare a 1/5 dilution of the cross-linker
reagent (supplied with the kit): add 10 micro litre of cross-linker reagent to
40 micro litre water. Place on ice until ready for use.
- Our probe DNA is prepared by PCR , followed
by gel extraction with a Qiagen kit. A typical DNA concentration of ~50 ng/micro
litre is obtained; therefore, 5 micro litre of gel-purified PCR product is
added to 20 micro litre water to obtain the proper amount and concentration of
DNA.)
- Heat the probe DNA (25 micro litre
for our typical case) in boiling water 5 min. or in a heat block at 100 C,
then on ice for 5 min., then spin down briefly.
- Add 25 micro litre of reaction buffer
(supplied with kit) to the denatured probe DNA, mix gently, then back on ice
(total volume = 50 micro litre).
- Add 5 micro litre of labeling reagent
(supplied with kit), mix gently, then back on ice.
- Add 25 micro litre for our typical case of
working dilution (1/5) of cross-linker reagent, mix gently, spin down contents
briefly.
- Incubate reaction mix for 30 min. in 37 C
water bath or heat block.
- Place probe on ice until ready for use (up
to 2 hours is fine).
- After prehybridization is complete (90 min.
prehyb. total), add PCR product labelled probe (~80 micro litre total)
directly to the hybridization buffer (best to remove hyb. solution to 50 ml
tube, add probe, mix briefly, then add back to the hybridization tube).
- Hybridize overnight in hybridization oven
at 60 C.
Post hybridization washes and blot development
- Preheat primary wash buffer (~1 liter) in a
water bath or microwave oven to 60 C. Prepare secondary wash buffer by adding
50 ml of 20X stock secondary buffer to 950 ml water and 2 ml of 1M MgCl2
solution. The secondary wash buffer is used at room temperature.
- Transfer blot from hybridization tube into
a clean plastic container with lid (DNA side up), wash two times in primary
wash buffer (500 ml, 60 C, 10 min. each wash).
- Transfer blot to clean plastic dish (DNA
side up), wash two times in secondary wash buffer (500 ml, room temperature, 5
minutes each wash).
- Remove blot from the secondary wash buffer,
and "wick off" excess buffer by touching a corner of the blot to the side of
the plastic dish (~10 sec.), then transfer blot DNA side up to a clean, dry
plastic dish (size similar to blot). Pipette ~8 ml (~0.025 ml/cm2)
of CDP-star reagent (provided with the kit) onto the blot, try to cover the
blot as evenly as possible. Tilt the dish with blot back and forth to
distribute the CDP-star to all areas of the blot, then incubate ~4 min. at
room temperature.
- Remove blot from CDP-star, "wick off"
excess CDP-star by touching the corner of the blot to the side of the plastic
dish (~10 sec.). Then place the blot inside a clean "sheet saver" plastic
sleeve (some trimming might be required) and then into a film cassette. The
blot will still be "shiney" wet with CDP-star when placing in the plastic
sleeve---this is normal. Drying or blotting on filter paper should not be
performed.
- Incubate the blot in the cassette for ~1
hr. at room temperature, then place a film on for 10-15 min., then develop.
Film exposure times may have to be varied to obtain the best results (we used
X-omat blue film for our test cases).

